Tolerability Profile
TRINTELLIX has an excellent tolerability profile1
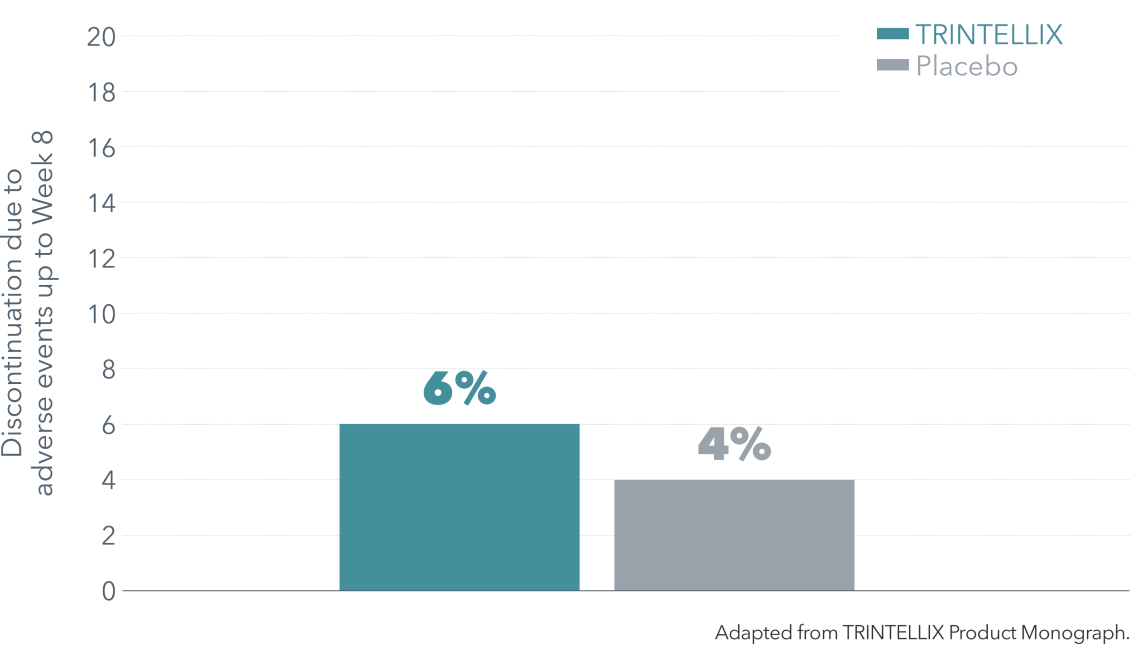
Discontinuation rates observed in short-term studies (up to Week 8)
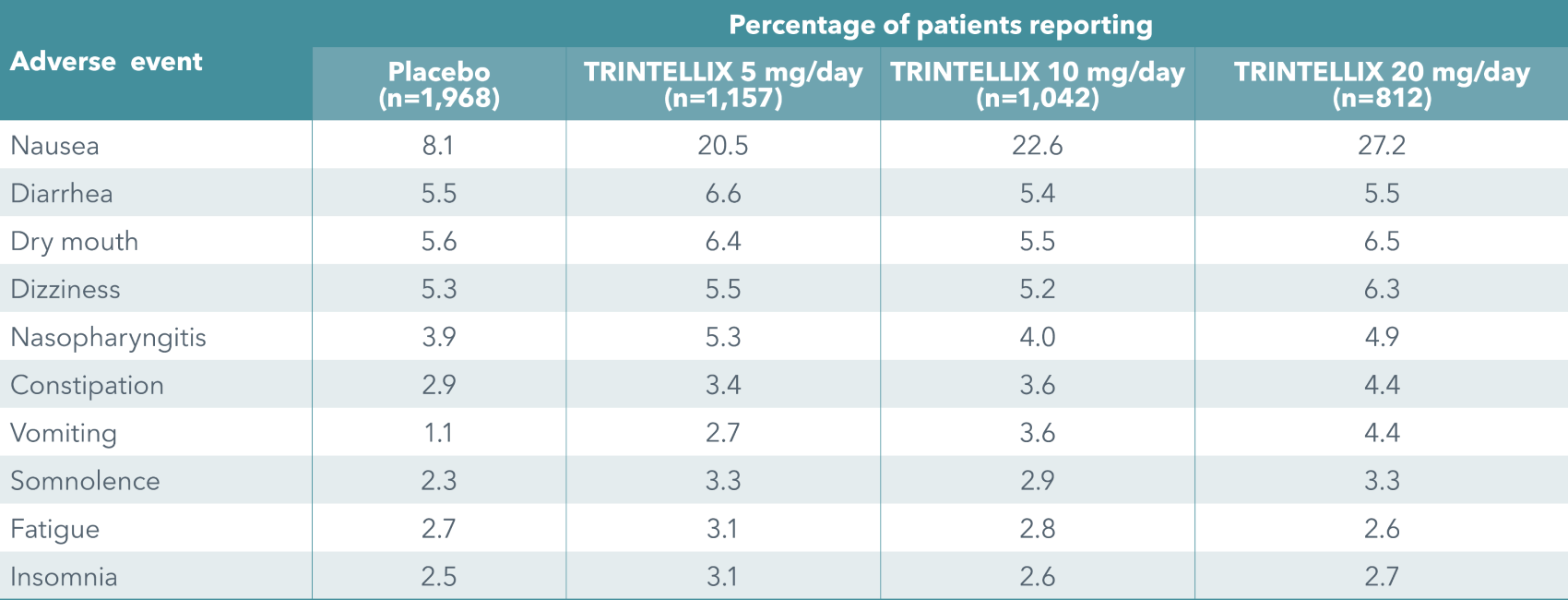
The most common reason for discontinuation of TRINTELLIX in short-term studies (up to 8 weeks) was nausea. The incidence of nausea leading to withdrawal was 1.1% (TRINTELLIX 5 mg), 1.4% (TRINTELLIX 10 mg), 3.8% (TRINTELLIX 15 mg) and 3.3% (TRINTELLIX 20 mg) vs 0.3% for placebo. Withdrawing due to nausea was highest during the initial weeks of treatment.1
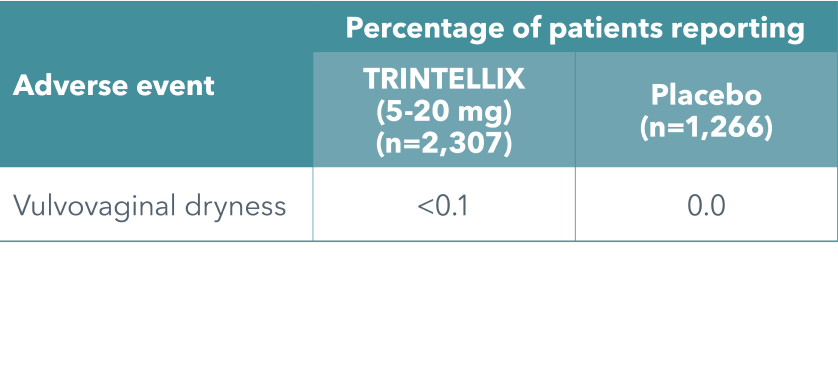
Low incidence of self-reported sexual side effects demonstrated1
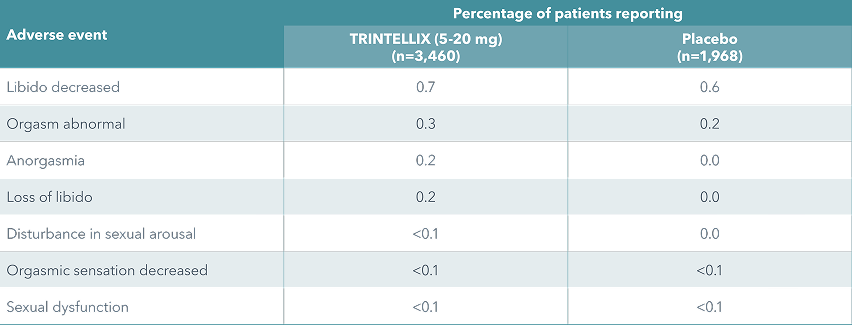
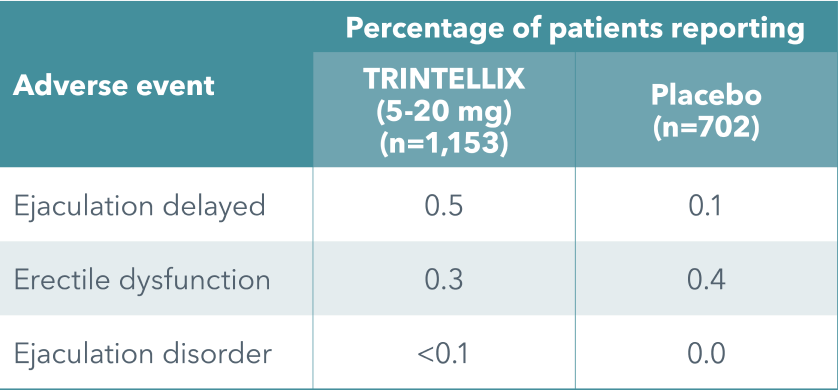
The incidence of self-reported sexual side effects was low and similar to placebo in clinical short- and long-term studies with TRINTELLIX (5-20 mg/day)
In males only:
In females only:
- In females: TRINTELLIX 5 mg/day 22% (N=65), 10 mg/day 23% (N=94), 20 mg/day 34% (N=67), placebo 20% (N=135)
- In males: TRINTELLIX 5 mg/day 16% (N=67), 10 mg/day 20% (N=86), 20 mg/day 29% (N=59), placebo 14% (N=162)
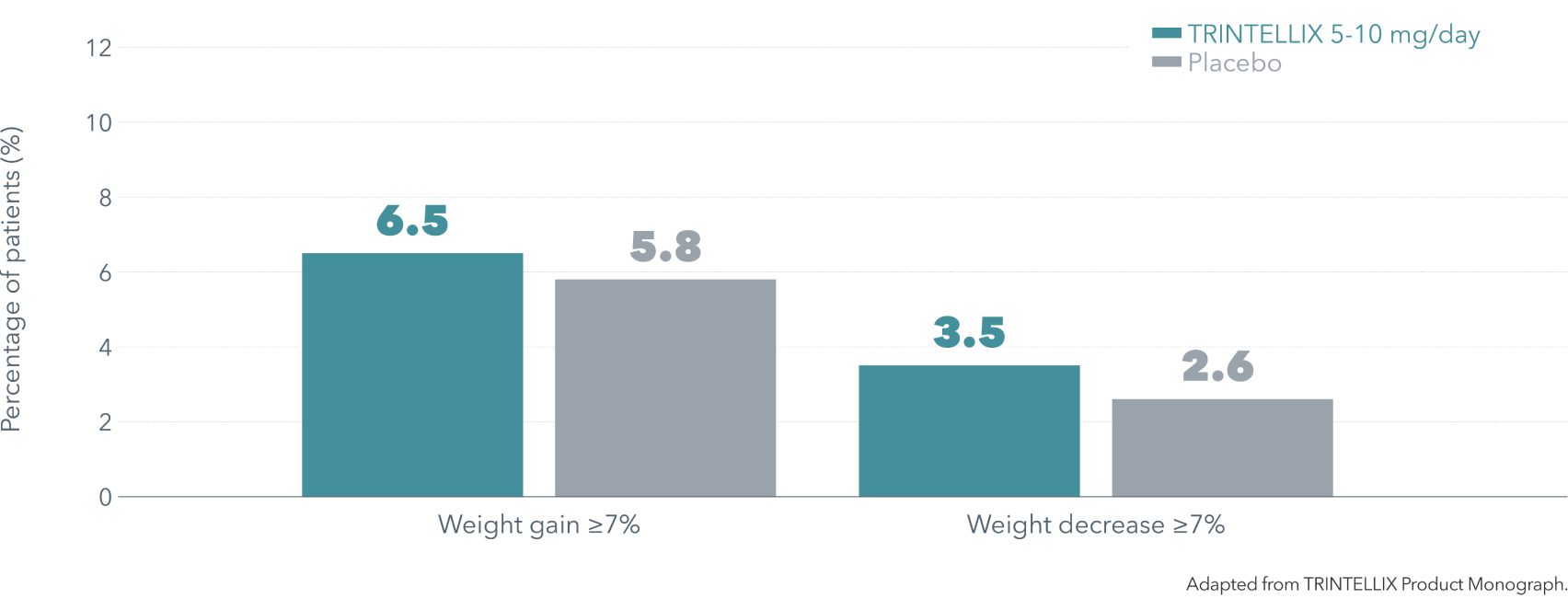
No clinically meaningful effect demonstrated on body weight1
Mean weight change from baseline in a long-term (24-64 weeks), placebo-controlled study: +0.4 kg for TRINTELLIX 5 or 10 mg/day, +0.1 kg for placebo.
No clinically significant effect demonstrated on ECG parameters1
TRINTELLIX has not been associated with any clinically significant effect on ECG parameters, including QT, QTc, PR and QRS intervals, or with any arrhythmogenic potential.
ECG=electrocardiogram; MDD=major depressive disorder
Reference: 1. TRINTELLIX Product Monograph. Lundbeck Canada Inc., May 27, 2024.