Efficacy Data
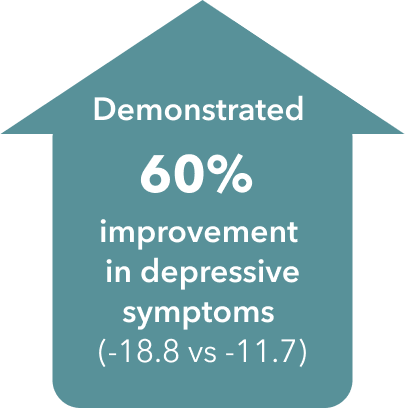
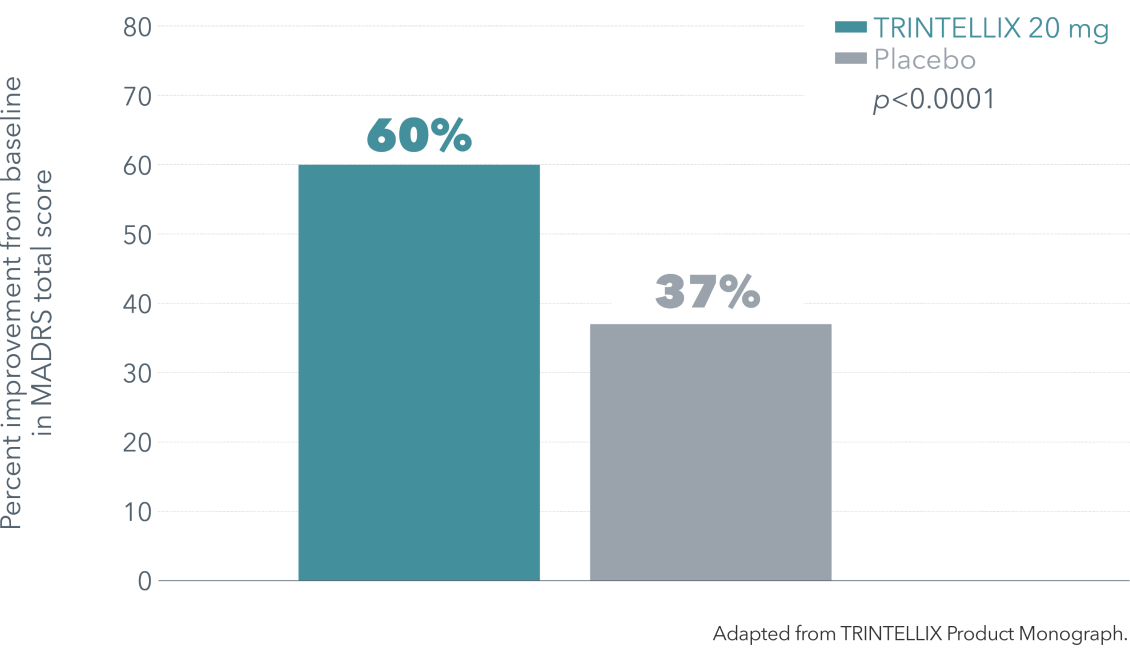
TRINTELLIX improved multiple symptoms of MDD1-3†‡§
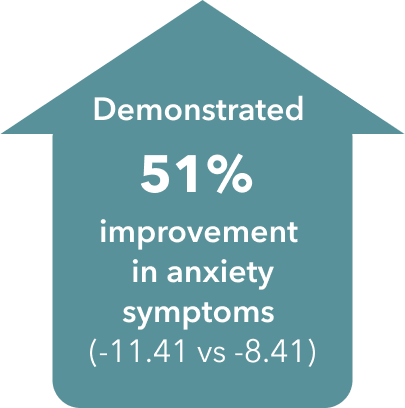
Anxiety Symptoms (HAM-A)
~90% of depressed patients are affected by anxiety symptoms7
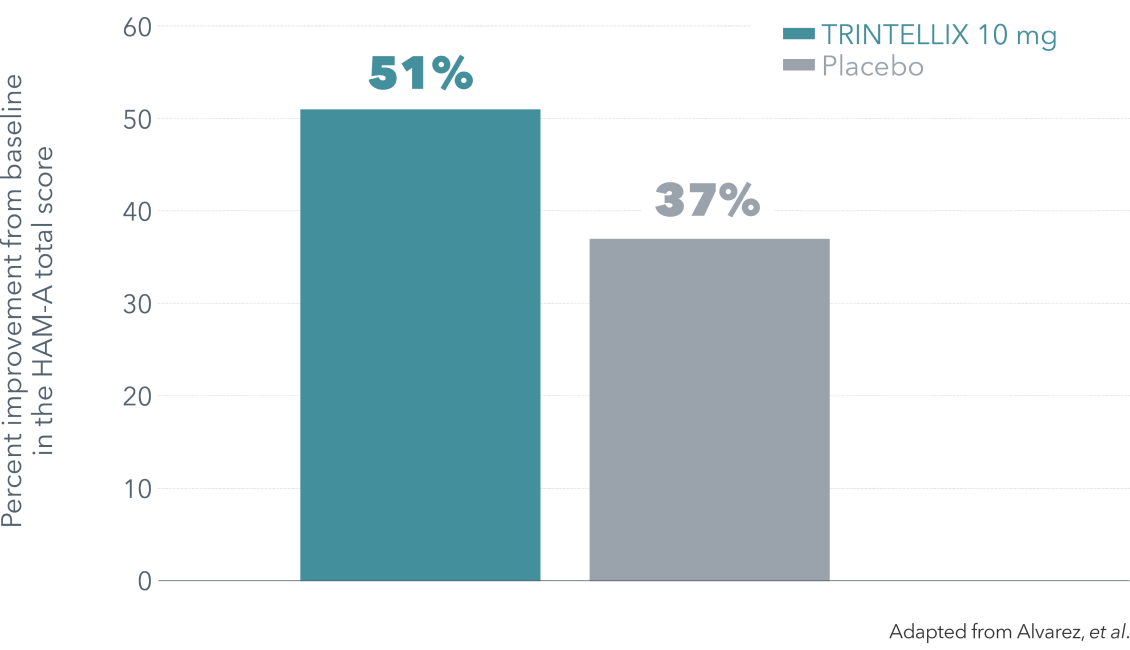
Improvement in anxiety symptoms vs placebo at Week 6
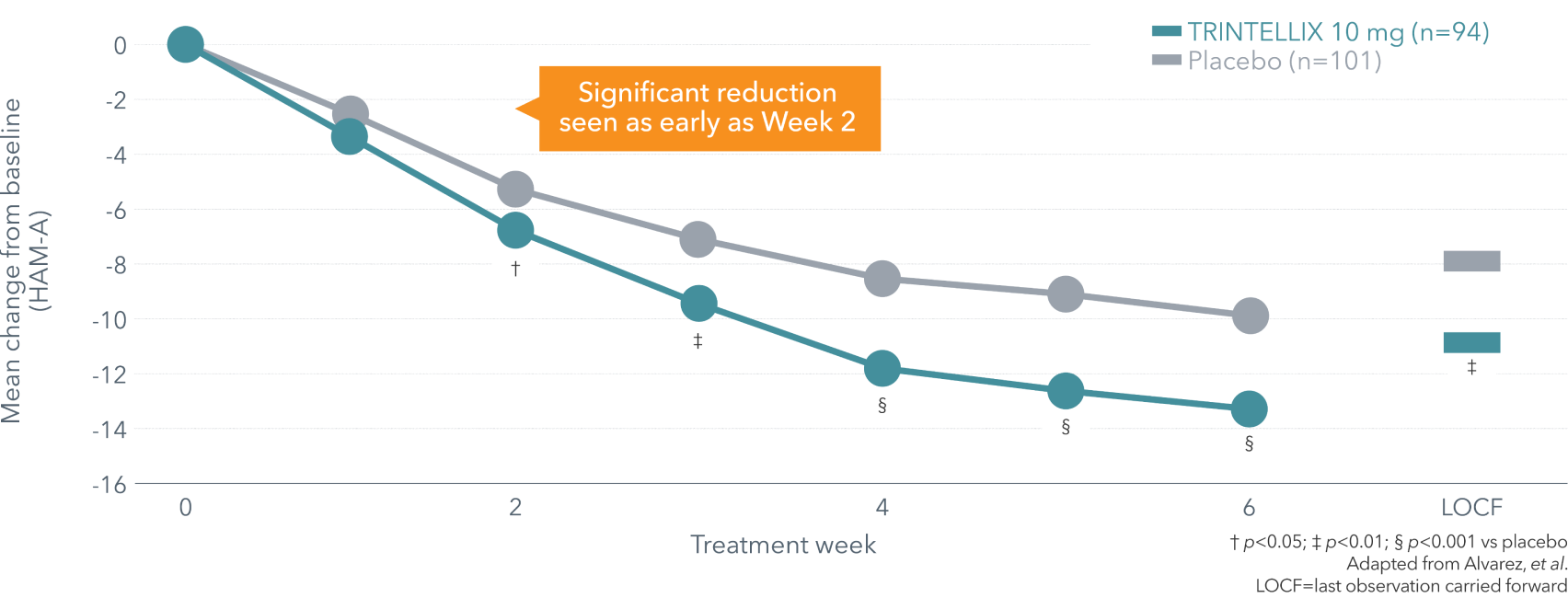
Improvement in anxiety symptoms vs placebo over 6 weeks (secondary endpoint)4¶
In a separate study, vortioxetine demonstrated similar effect to venlafaxine XR in Asian patients on HAM-A total score.
Mean reduction (-11.4 vs -10.6) from baseline at 8 weeks with TRINTELLIX 10 mg vs venlafaxine XR 150 mg (95% CI: -2.09 to 0.45; secondary endpoint)5||
Overall Function (SDS)
Demonstrated improvement in overall function from baseline at 8 weeks with TRINTELLIX 20 mg vs placebo1†‡
Up to 87% improvement in overall function (-8.4 vs -4.5, p=0.0005)1†‡
TRINTELLIX improved function in family, work and social life in patients with MDD (secondary endpoints)1†‡
Up to 86% improvement (-2.6 vs -1.4) in function at work (p=0.0059)1†‡
Up to 82% improvement (-3.1 vs -1.7) in function at home (p<0.0001)1†‡
Up to 82% improvement (-3.1 vs -1.7) in function in a social setting (p<0.0001)1†‡
†The starting and recommended dose of TRINTELLIX is 10 mg once daily for adults <65 years.
See Product Monograph for complete dosing and administration information.
CANMAT=Canadian Network for Mood and Anxiety Treatments; CGI-S=Clinical Global Impression–Severity; CI=confidence interval; DSM-IV-TR=Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision; DSST=Digit Symbol Substitution Test; HAM-A=Hamilton Anxiety Rating Scale; MADRS=Montgomery-Åsberg Depression Rating Scale; MDD=major depressive disorder; MDE=major depressive episode; SDS=Sheehan Disability Scale
‡ Double-blind, fixed-dose, placebo-controlled study of 608 patients aged 18-75 years with a primary diagnosis of recurrent MDD according to DSM-IV-TR criteria, a current MDE >3 months’ duration and a MADRS total score ≥26. Patients were randomized to TRINTELLIX 15 mg, 20 mg (10 mg/day during Weeks 1 and 15 or 20 mg/day from Weeks 2 to 8) or placebo for 8 weeks. Mean baseline MADRS total scores were 31.5 for placebo, 31.8 for TRINTELLIX 15 mg and 31.2 for TRINTELLIX 20 mg. Mean baseline SDS total scores were 19.8 for placebo, 20.6 for TRINTELLIX 15 mg and 20.7 for TRINTELLIX 20 mg. Mean baseline SDS work scores were 6.3 for placebo, 6.8 for TRINTELLIX 15 mg and 6.9 for TRINTELLIX 20 mg. Mean baseline SDS social scores were 6.8 for placebo, 6.9 for TRINTELLIX 15 mg and 6.8 for TRINTELLIX 20 mg. Mean baseline SDS family scores were 6.9 for placebo, 6.7 for TRINTELLIX 15 mg and 7.0 for TRINTELLIX 20 mg.
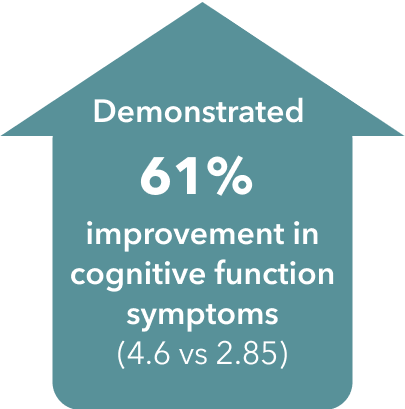
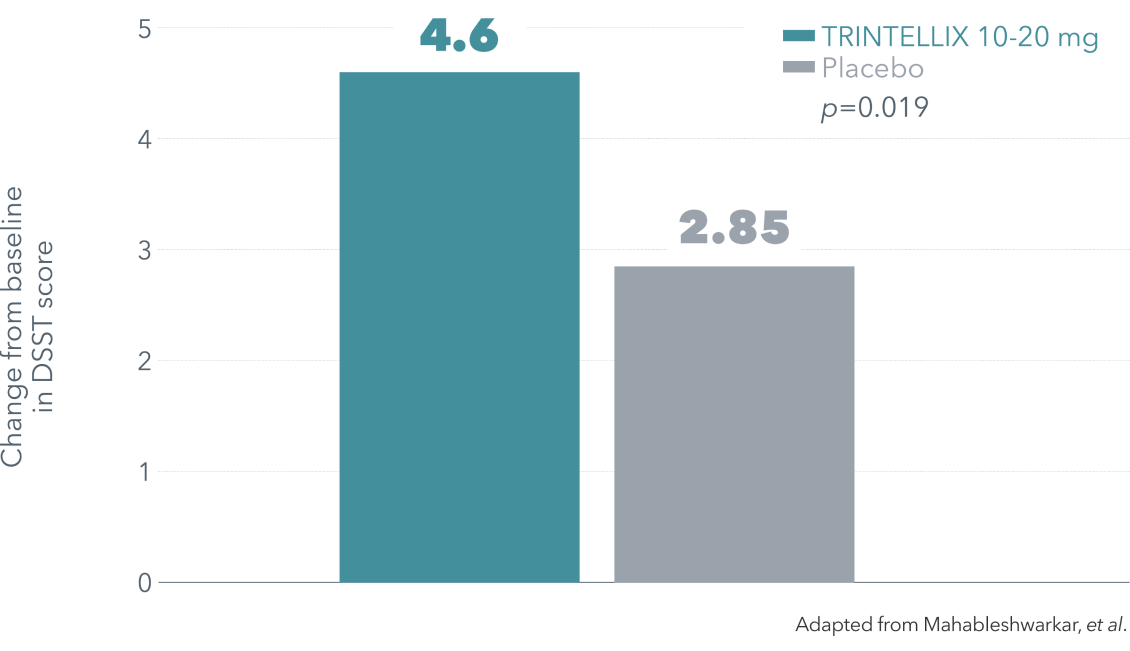
§ Double-blind, parallel-group, placebo-controlled, active-reference study of 602 patients aged 18-65 years with a DSM-IV-TR diagnosis of MDD, a current MDE ≥3 months’ duration, a MADRS total score ≥26, in addition to self-reported subjective cognitive dysfunction with a baseline score of <70 on the DSST. Patients were randomized to TRINTELLIX 10 or 20 mg once daily (n=198) or placebo (n=194) for 8 weeks. Duloxetine 60 mg once daily (n=207) was included as the active reference arm to demonstrate assay sensitivity to traditional antidepressant outcomes. Mean baseline DSST scores were 43.5 for placebo, 42.3 for TRINTELLIX 10-20 mg and 43.4 for duloxetine 60 mg.
¶ Double-blind, fixed-dose, placebo-controlled, active reference study of 429 patients aged 18-65 with MDD presenting with a current MDE according to DSM-IV-TR criteria and a MADRS total score ≥30 at baseline. Patients were randomized to TRINTELLIX 5 or 10 mg for 6 weeks or placebo. Mean baseline HAM-A total scores were 22.9 for placebo and 22.3 for TRINTELLIX 10 mg.
|| Double-blind, fixed-dose study of 443 Asian patients aged 18-65 years with recurrent MDD presenting with a current MDE according to DSM-IV-TR criteria, a MADRS total score of ≥26 and a CGI-S score ≥4 at screening and at baseline. Following a screening period of up to 7 days, eligible patients were randomized to TRINTELLIX 10 mg/day or venlafaxine XR 150 mg/day (75 mg/day for the first 4 days of treatment) for 8 weeks. The primary analysis tested for non-inferiority of TRINTELLIX and venlafaxine XR using the change from baseline in MADRS total score at Week 8 based on the FAS. MADRS response was defined as a ≥50% decrease in MADRS score from baseline and remission was defined as a MADRS total score ≤10.
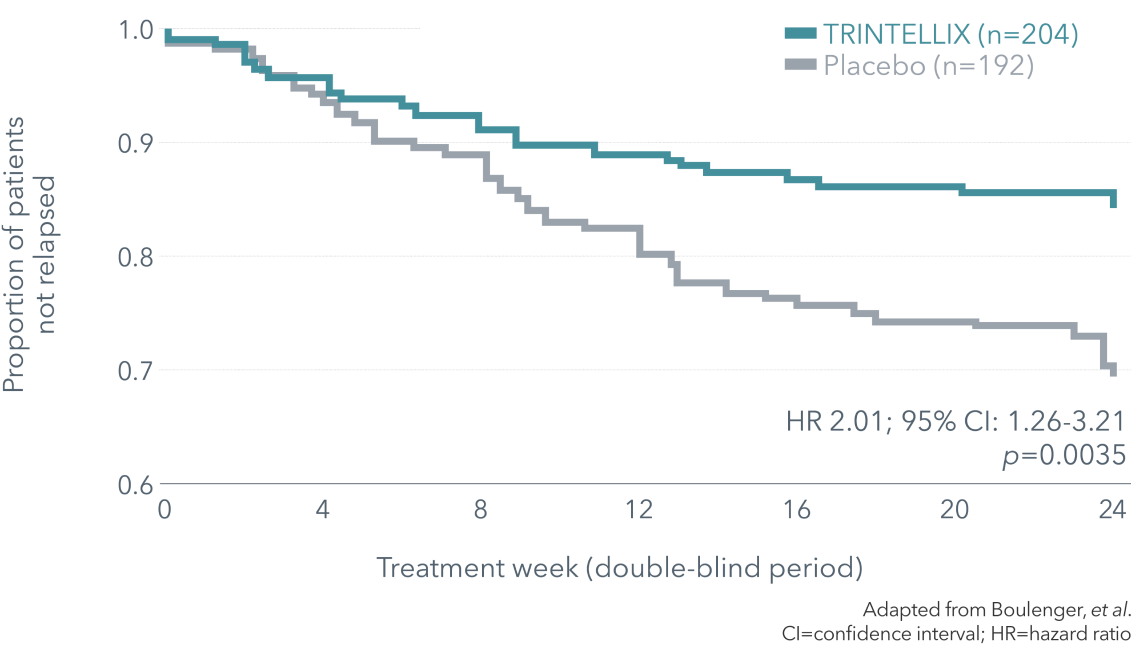
** Double-blind, randomized, placebo-controlled, relapse prevention study of 639 in- and outpatients aged 18-75 with a primary diagnosis of MDD according to DSM-IV-TR criteria presenting with a MDE of ≥4 weeks in duration and ≥1 prior MDE and a MADRS total score ≥26 at baseline. The study consisted of a 12-week, open-label, flexible-dose treatment period with TRINTELLIX 5-10 mg/day. Patients in remission (MADRS total score ≤10) at both Weeks 10 and 12 were randomized to the double-blind, placebo-controlled, fixed-dose treatment period and received either TRINTELLIX (the final dose [5 or 10 mg] that was fixed from Week 8 in the open-label period) or placebo. For approximately 70% of randomized patients, the dose of TRINTELLIX at the end of the open-label period and during the double-blind period was 10 mg/day. Relapse was defined as a MADRS total score ≥22 or an insufficient therapeutic response, as judged by the investigator.
References: 1. Boulenger JP, et al. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol 2014;29(3):138-49. 2. TRINTELLIX Product Monograph. Lundbeck Canada Inc., May 27, 2024. 3. Mahableshwarkar AR, et al. A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology 2015;40(8):2025-37. 4. Alvarez E, et al. A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol 2012;15(5):589-600. 5. Wang G, et al. Comparison of vortioxetine versus venlafaxine XR in adults in Asia with major depressive disorder: a randomized, double-blind study. Curr Med Res Opin 2015;31(4):785‑94. 6. Boulenger JP, et al. A randomized clinical study of Lu AA21004 in the prevention of relapse in patients with major depressive disorder. J Psychopharmacol 2012;26:1408-16. 7. Sadock BJ, et al. Depression and bipolar disorder. In: Kaplan & Sadock’s Concise Textbook of Clinical Psychiatry. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2008. 8. Lam RW, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2023 Update on Clinical Guidelines for Management of Major Depressive Disorder in Adults. Can J Psychiatry 2024:1-47.